Site Initiation Visit Confirmation Letter
Confirm that you are showing up as agreed.
Site initiation visit confirmation letter. All sites must undergo a siv prior to the ci activating the site to start the trial site activation. Site initiation activation and close out sop. Identifying critical suppliers sop.
How to format the letter. Site visit forms site initiation visit confirmation letter template 10 1 2005 site initiation visit follow up letter template 10 1 2005 site initiation visit sample agenda 10 1 2005. Nci pi name the lead investigator for this protocol.
Report forms safety monitoring committee smc report template march 2014. Pi name this letter is to confirm that i will be at your site on date and time for the site initiation visit for protocol title. Ci perform site initiation visits siv at each site train site staff resolve all issues and complete reports.
Site assessment questionnaire template. Site selection visit form. Before your visit you will coordinate a convenient time for the study site and confirm your visit with a letter informing them when you will arrive and what the objectives of the visit will be.
Sop s 1011 appendix 7 non ctimp site initiation checklist and outstanding issues report non ctimp version 1 march 2016 uol site initiation checklist for studies not involving investigational medicinal products 1 site information site initiation visit method sponsor reference number. You ll send a confirmation letter or email if your sops allows it prior to every monitoring visit be it a pre study qualification visit a site initiation visit routine monitoring visit close out visit etc. I expect the visit to last approximately hours.
I will be attending on behalf of with dr. Then you ll need to document your visit findings in a monitoring report. Site feasibility tracker.
Site initiation visit for protocol title dear dr. Archiving trial data sop. Ci pi confirmation of review and compliance with.
Site assessment and feasibility questionnaire. Site selection visit checklist. Site visit log 10 1 2005 telephone log 10 1 2005.
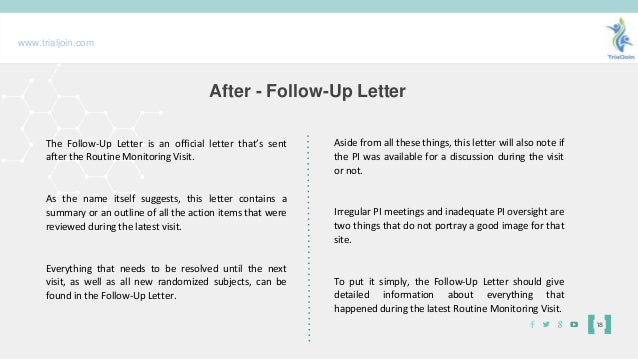
Finally you will send the principal investigator a follow up letter summarizing the visit and discussing any critical findings or action items. Start by referencing the original correspondence when the visit agreement was made. The meeting will last for approximately x hours.
It is important to physically be at the site so as a monitor you can visit the labs pharmacies and other areas where the research will be conducted to ensure they are adequate. Use a visit confirmation letter when you want to confirm your visit to a company on a previously agreed date and time. Planned start to end date dear dr.